electronic Disease Activity Index
for PSoriatic Arthritis
e
eDAPSA
Psoriasis arthritis assessment
made easy and quick
Android version
(coming soon)
Illustrative PDF
(coming soon)
Original PDF
(coming soon)
What is the eDAPSA?
The Disease Activity index in PSoriatic Arthritis (DAPSA) is used to assess disease activity in patients with confirmed psoriatic arthritis (PsA). The score has become established, particularly in Europe, and is recommended by international experts as a measurement tool for assessing disease activity in PsA. The DAPSA index includes laboratory parameters, clinical aspects, and patient self-assessments.
Validation
paper version: yes digital version: no
How to use the eDAPSA
The DAPSA is based on four pillars:
- number of joints with pressure pain (0 - 68)
- number of swollen joints (0 - 66)
- C-reactive protein (CRP) (mg/l)
- patient assessment of disease activity and pain.
To calculate the DAPSA or cDAPSA index, the values given above are added together in a sum score.
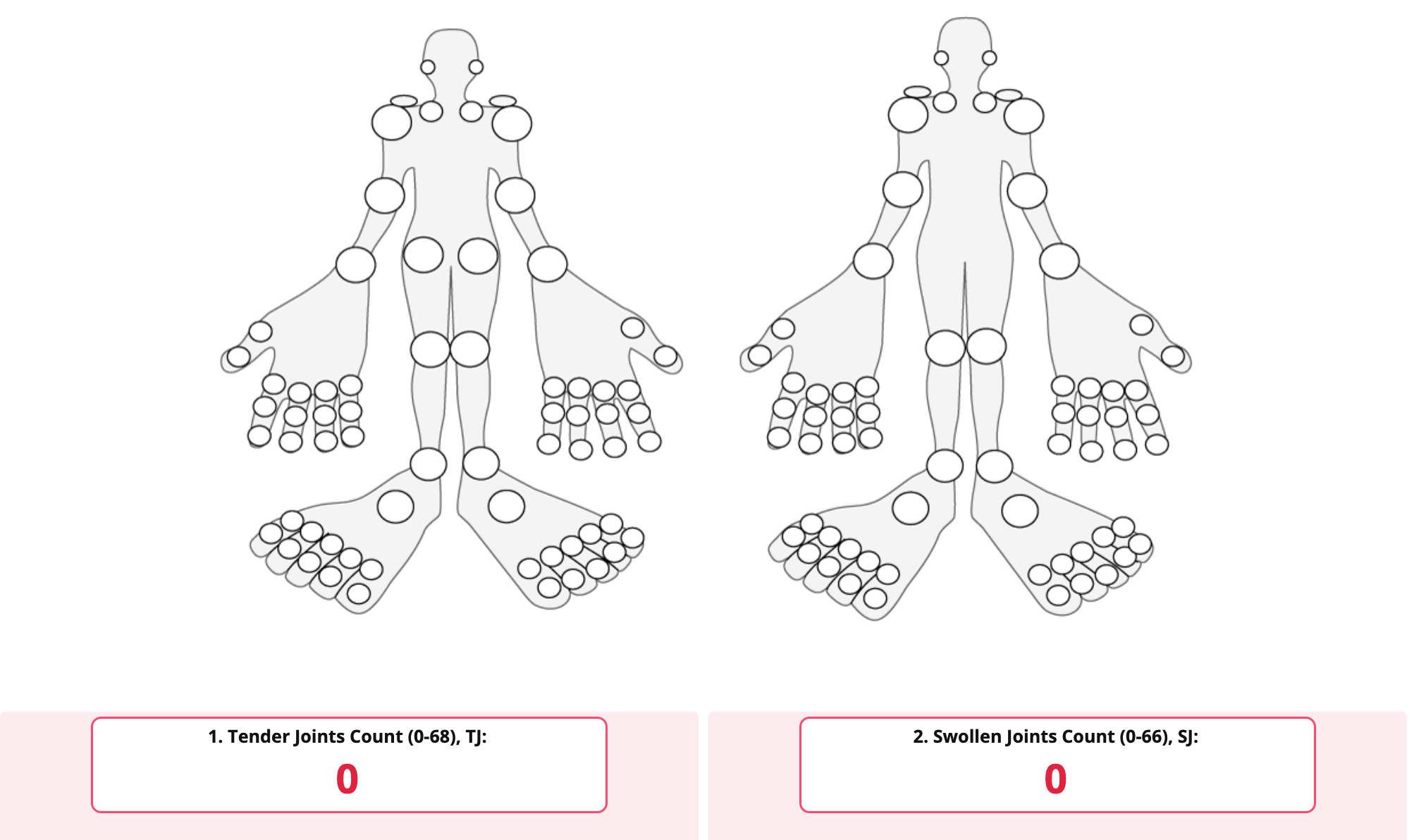
For the DAPSA:
Pressure painful joints (0 - 68) + swollen joints (0 - 66) + C-reactive protein (mg/l) + disease activity + pain.
To determine disease activity, the following values apply:
- 0 - 4 remission
- 5 - 14 low disease activity
- 15 - 28 medium disease activity
- >28 high disease activity
For the cDAPSA:
Pressure painful joints (0 - 68) + swollen joints (0 - 66) + disease activity + pain.
To determine disease activity, the following values apply:
- 0 - 4 remission
- 5 - 13 low disease activity
- 14 - 27 medium disease activity
- >27 high disease activity
The following versions of the eDAPSA are available
Android version
(coming soon)
Illustrative PDF
(coming soon)
Original PDF
(coming soon)
How was the eDAPSA developed?
The DAPSA was developed by Professor Dr. Smolen and colleagues at the University Hospital in Vienna with the participation of international experts and validated based on clinical data.
To make the DAPSA applicable in clinical practice, a clinical version (cDAPSA) has been developed which can be used independently of inflammatory markers. In validation studies, a high agreement of the cDAPSA and DAPSA could be demonstrated.
Features
USER-FRIENDLY
The DAPSA can now be filled out quickly and easily online.
ENVIRONMENTALLY FRIENDLY
Take care of the environment! The simple DAPSA form can be filled out online and easily saved to your records, eliminating un-necessary printouts.
DOCUMENTATION
Document proriasis arthritis activity and save the results or forward them to your patient.
VALIDATED
The DAPSA is validated and has already been applied in many international studies.
References
Validation:
Schoels, M., Aletaha, D., Funovits, J., Kavanaugh, A., Baker, D., & Smolen, J. S. (2010). Application of the DAREA/DAPSA score for assessment of disease activity in psoriatic arthritis. Annals of the rheumatic diseases, 69(8), 1441–1447. https://doi.org/10.1136/ard.2009.122259
Smolen, J. S., Schoels, M., & Aletaha, D. (2015). Disease activity and response assessment in psoriatic arthritis using the Disease Activity index for PSoriatic Arthritis (DAPSA). A brief review. Clinical and experimental rheumatology, 33(5 Suppl 93), S48–S50.
van Mens, Leonieke J. J.; van de Sande, Marleen G. H.; van Kuijk, Arno W. R.; Baeten, Dominique; Coates, Laura C. (2018): Ideal target for psoriatic arthritis? Comparison of remission and low disease activity states in a real-life cohort. In: Annals of the rheumatic diseases 77 (2), S. 251–257. DOI: 10.1136/annrheumdis-2017-211998
Minimal Clinically Important Difference (MCID):
Karmacharya, P., Stull, C., Stephens-Shields, A., Husni, M. E., Scher, J. U., Craig, E., Fitzsimmons, R., Reddy, S. M., Magrey, M. N., Ogdie, A., & Walsh, J. A. (2023). Responsiveness and Minimum Clinically Important Difference in Patient-Reported Outcome Measures Among Patients With Psoriatic Arthritis: A Prospective Cohort Study. Arthritis care & research, 75(10), 2182–2189. https://doi.org/10.1002/acr.25111
Our other tools
Our partners





